
IPSNEWS

Korea Sourcing Week Case Study / Caso de Estudio
The Korea SMEs and Startups Agency (KOSME), a key government entity, sought to bridge the gap between innovative Korean cosmetic manufacturers and the lucrative Mexican market. They partnered with Insumos para la Salud (IPS) to facilitate meaningful connections at the Korea Sourcing event.

Key considerations for Cosmetic and Dietary Supplement claims in Mexico
Mexican regulations establish fundamental principles governing claims for cosmetic products and dietary supplements. The main objective is to ensure that these products are safe, effective, and not misleading to consumers.

Keys to the Labeling of Dietary Supplements in Mexico
Dietary supplements are defined as products intended to increase or complement the total dietary intake, or to supply one or more of their components. COFEPRIS is the authority in Mexico responsible for regulating these products and for establishing the labeling requirements they must meet to be marketed in the country.

Convocatoria para asistir a Korea Sourcing Week - 2025
Este año, IPS colaborará estrechamente con Korea SMEs and Startups Agency (KOSME) para convocar e identificar a dos grandes compradores mexicanos del sector cosmético interesados en participar en Korea Sourcing Week 2025, que se llevará a cabo del 3 al 6 de septiembre en Ilsan, Corea del Sur.

Classification Query: A Regulatory Tool for Marketing Your Cosmetics and Dietary Supplements
The classification query submitted to the Federal Commission for the Protection against Sanitary Risks (COFEPRIS) is a crucial step in regulating cosmetic products and dietary supplements.

Cosmetic Product Labeling in Mexico: Essential Requirements
In Mexico, cosmetic product labeling is regulated to ensure consumers receive accurate and sufficient information, enabling them to make safe and informed purchasing decisions. Compliance with these regulations is essential for manufacturers and importers looking to sell their products in the country.

Import Permit for Products: Requirements for Dietary Supplements
To import dietary supplements into Mexico, it is essential to understand the applicable regulations, among which the prior sanitary import permit stands out.
This permit, issued by COFEPRIS, authorizes the entry of food, dietary supplements, and non-alcoholic beverages that comply with Mexico’s current sanitary legislation, allowing their commercialization and distribution in the country.

IPS Joins CANIPEC: Empowering innovation and regulatory excellence in Mexico’s cosmetics industry
Insumos para la Salud (IPS) proudly announces its membership in the National Chamber of the Cosmetics Industry (CANIPEC), strengthening its commitment to sustainable growth, innovation, and driving clear and effective regulations in Mexico’s vibrant cosmetics and personal care sector.

Importation of Cosmetics and Dietary Supplements in Mexico: Key Aspects
The importation of cosmetic products and dietary supplements into Mexico requires compliance with health and customs regulations, the objective of which is to protect consumer health.
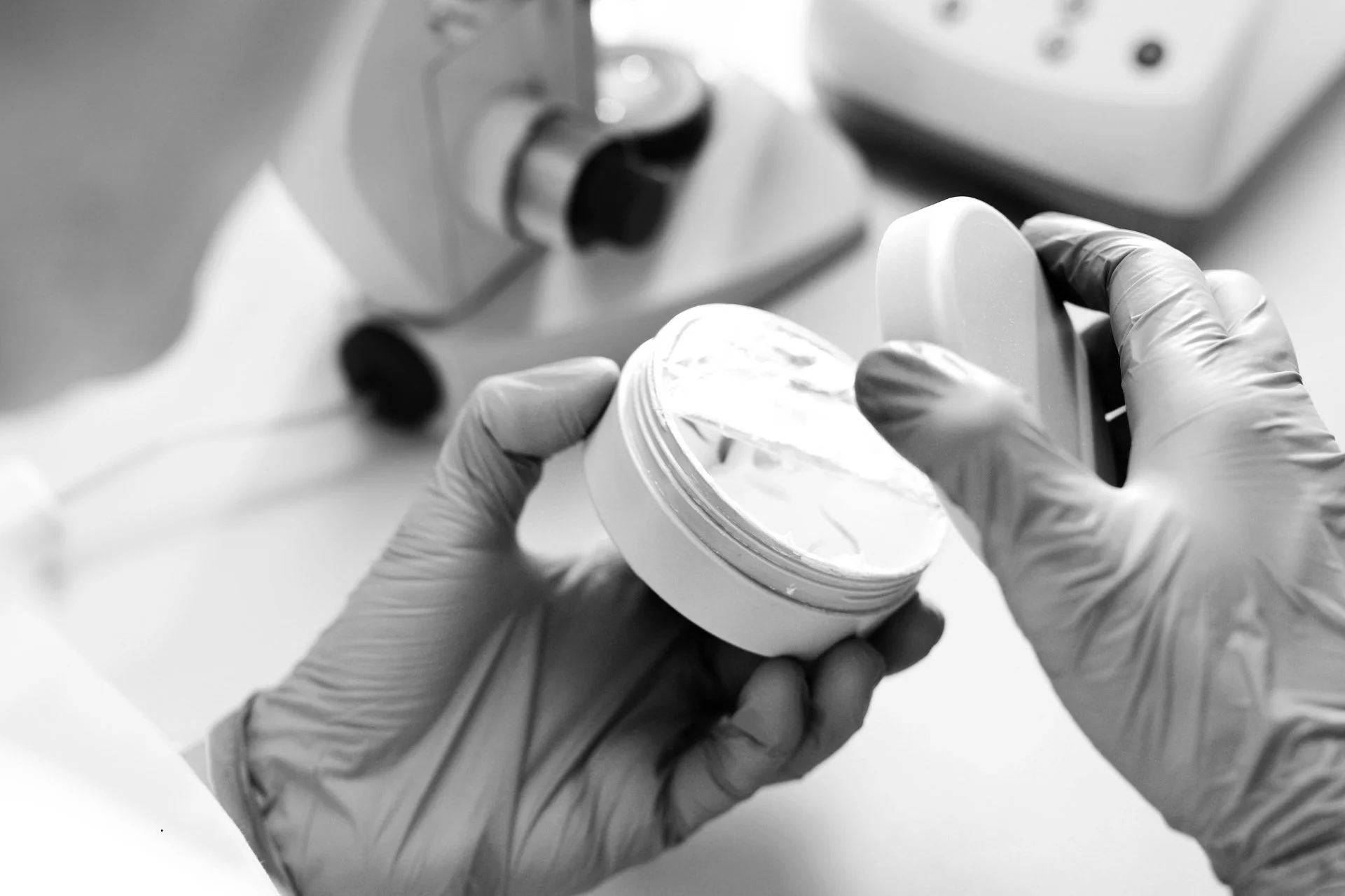
Essential Tests for Cosmetics in Mexico: Ensuring Safety and Quality
In Mexico, the safety and quality of cosmetic products are regulated by strict standards. Laboratory tests are essential to ensure that cosmetics meet established standards and protect consumer health. This article details the essential tests required, based on Mexican regulations.
